Intended Uses
3D Cage Cervical is a medical device used in surgical treatment of cervical fusion for patients with cervical disc disorders within the C2 to C7 range.
Shape and Structure
This product is an intervertebral fusion implant used in surgical treatment of cervical spine disorders for cervical fusion procedures
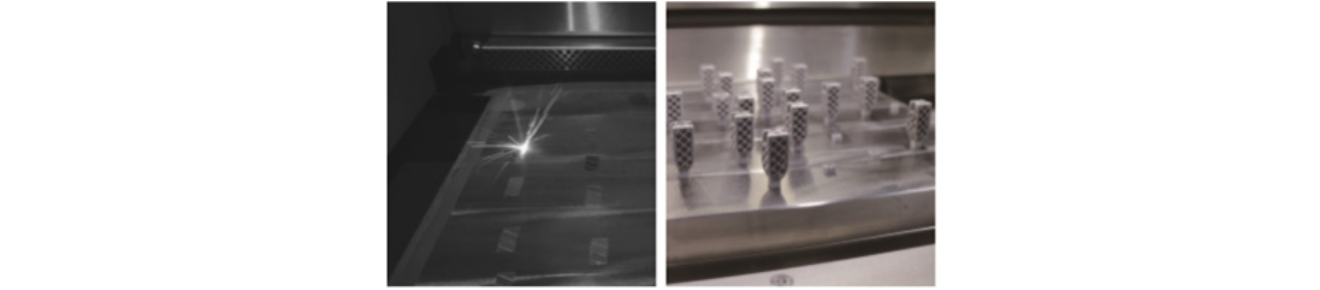
It is manufactured using 3D printing technology, specifically the Selective Laser Sintering (SLS) method, utilizing Titanium Powder.
| Type |
Name |
Product Appearance |
| 1 |
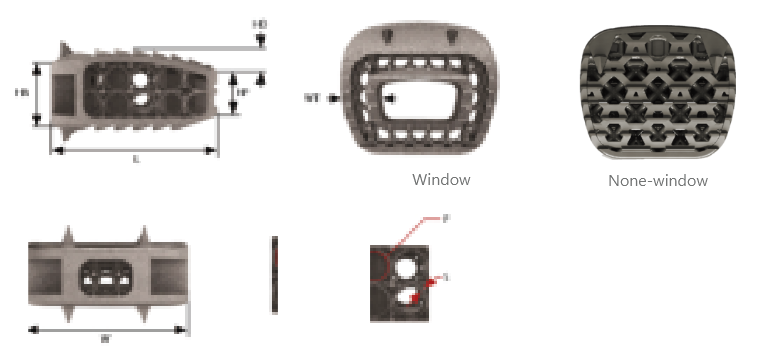
Body |
It is an implant made of Ti-6Al-4V ELI material and consists of a mesh structure and an outer frame. |
| 2 |
Keel |
It is a tooth-shaped structure designed to prevent dislodgment after device insertion. |
| 3 |
Window |
It is an empty space in the axial direction designed for bone grafting material placement. |
| 4 |
Spike |
It aids in the initial fixation after device insertion. |
| 5 |
Instrumentation Attachment |
It is the area where surgical instruments are attached for device insertion. |
Specifications
The SLS Technique 3D Cage™ is produced using the Selective Laser Sintering (SLS) technology.
Model Name
| Product |
HA(mm) |
WA(mm) |
HD(mm) |
HP(mm) |
W(mm) |
L(mm) |
S(Ø) |
WT(mm) |
| CC |
3~15 |
0~1 |
3~13 |
10~20 |
11~18 |
0~1.2 |
0.7~1.2 |
3~10 |