Features
- Contains Lidocaine as local aesthetic
- Wrinkles with crosslinked hyaluronic acid particles temporarily
- Made with pharmaceutical grade hyaluronic acid
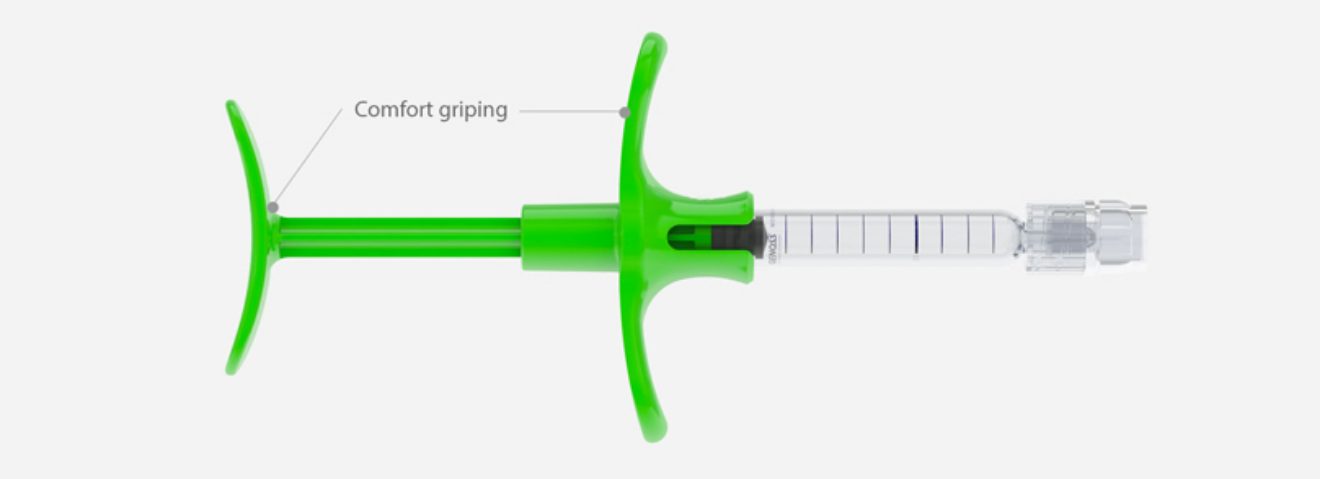
Convenience
Ergonomically designed grips and push rods provide accurate and safe treatment with constant injection forces.
It allows the user to perform various injection technique according to treatment site.
Safety
MONALISA minimized side effects through a strict quality control system.
|
Standard |
Result |
| Appearance |
No impurities, transparent and colorless gel |
Pass |
| HA² |
21.6 ~ 26.4mg/mL |
24.1mg/mL |
| pH |
6.5 ~ 7.5 |
7.10 |
| Residual BDDE |
< 2ppm |
Not Detected/td>
|
| Endotoxin |
< 0.5 EU/mL |
< 0.1 EU/mL |
| Volume |
> 1.0mL |
Pass |
* What is BDDE(1,4-butanediol diglycidyl ether, cross-linking agent)?
BDDE(1,4-butanediol diglycidyl ether) is a chemical cross-linking agent to make hyaluronic acid into gel form.
The residual amount of BDDE is strictly regulated to less than 2ppm. It was not detected in Every MONALISA Filler.
Model Name
| Model Name |
Particle Size |
Capacity |
Concentration of Lidocaine |
| MPF10S |
200㎛ |
1.0ml |
0.3% |
| MPF10M |
400㎛ |
| MPF10H |
600㎛ |
| MPF10U |
900㎛ |